A trial looking at adding radiotherapy to atezolizumab for people with urothelial (urinary tract) cancer that has spread (RE-ARM)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is for people with urothelial (urinary tract) cancer that has spread to somewhere else in the body.
It is looking at adding radiotherapy to a drug called atezolizumab. The team want to find out if it works better than ateolizumab on its own.
Cancer Research UK supports this trial.
More about this trial
Atezolizumab is a treatment for some people with urinary tract cancer that has spread to another part of the body. This is also called metastatic, secondary or advanced cancer.
The urinary tract includes the:
- centre of the kidney (renal pelvis)
- tube that takes urine from the kidney to the bladder (ureter)
- bladder
- tube that drains urine from the bladder and out of the body (urethra)
The lining of the urinary tract is called the urothelium, so cancer of the urinary tract can also be called urothelial cancer.
Atezolizumab is a type of immunotherapy. It helps to make your  find and kill cancer cells.
find and kill cancer cells.
Radiotherapy uses high energy x-rays to kill cancer cells. We know from research that radiotherapy can sometimes help our immune system’s response to cancer. Researchers think that this might mean it could help atezolizumab work better.
Atezolizumab usually starts to work in the first few weeks of treatment. But for some people in the first few weeks their cancer stays the same or gets worse. For some of these people, the atezolizumab starts to work later on. This is called a late response. The trial team think adding radiotherapy to atezolizumab could increase the number of people having a late response.
Avelumab is another type of immunotherapy. It can help to delay urinary tract cancer from getting worse after chemotherapy. Having avelumab in this way is called maintenance therapy. But it does not work for everyone. Researchers think that stopping avelumab when it is not working and starting atezolizumab might help some people with urinary tract cancer, but they are not sure. Part of this trial is to try and find this out.
The aims of the trial are to:
- see if adding radiotherapy to atezolizumab is better than just atezolizumab alone
- see if stopping avelumab and starting atezolizumab helps some people with urinary tract cancer
- learn more about side effects of treatment
- find out more about the quality of life of people taking part
Who can enter
The following bullet points are a summary of the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
Who can take part
You may be able to join this trial if all of the following apply. You:
- have cancer that started in the layer of cells that line most of the
urinary system  (urothelial cancer). If you have a mixed type of cancer you might still be able to take part, if most of the cancer cells are urothelial. Your doctor can explain more.
(urothelial cancer). If you have a mixed type of cancer you might still be able to take part, if most of the cancer cells are urothelial. Your doctor can explain more. - have urothelial cancer that has spread to somewhere else in the body
- have cancer that your doctors do not think will go away completely with just surgery or radiotherapy
- have had up to 6 months of treatment with atezolizumab or maintenance avelumab (with certain chemotherapy drugs) and your cancer has grown. Your doctor can explain more.
- have had between 3
cycles of treatment  and 6 months of treatment with atezolizumab and your cancer has either grown or
and 6 months of treatment with atezolizumab and your cancer has either grown or stayed the same  . Your doctor can explain more.
. Your doctor can explain more. - have cancer in an area that is suitable for radiotherapy. This must be outside the brain. Your doctor can explain more.
- have cancer in another area away from where you will have radiotherapy
- have satisfactory blood test results
- are fully active or you can't carry out heavy physical work, but can look after yourself and do anything else (ECOG Performance Status 0-1)
- are willing to use reliable contraception during treatment and for around 5 months after if there is any possibility that you or your partner could become pregnant. Your doctor will let you know about suitable contraception.
- are at least 18 years old
Who can’t take part
You cannot join this trial if any of these apply:
- you have had radiotherapy during the 4 weeks before starting atezolizumab or avelumab before joining the trial
- you have had radiotherapy while having atezolizumab or avelumab before joining the trial
- it has been longer than 8 weeks since your last dose of atezolizumab or maintenance avelumab
- you have had over 6 months of treatment with atezolizumab or maintenance avulumab before
randomisation 
- you have had another very similar immunotherapy drug before starting atezolizumab or maintenance avelumab
- you have had atezolizumab with
chemotherapy  in the past
in the past - your doctor thinks that continuing atezolizumab might not be suitable for you, for example if the side effects are too bad or it would be better for you to have a different type of treatment, such as chemotherapy
- you are likely to need radiotherapy to help with your cancer symptoms in the 9 weeks after joining the trial
- you have had a drug called a checkpoint inhibitor before starting atezolizumab or maintenance avelumab. Your doctor can explain more.
- you have had treatment to dampen down your immune system (
immunosuppression  apart from low doses of
apart from low doses of steroids  during the 2 weeks before
during the 2 weeks before randomisation) 
- it wouldn’t be safe for you to have radiotherapy
- you have an
autoimmune disease  and are needing immunotherapy treatment or are having life threatening complications. You can still take part if you have vitiligo, psoriasis that is under control, autoimmune thyroid disease or type 1 diabetes.
and are needing immunotherapy treatment or are having life threatening complications. You can still take part if you have vitiligo, psoriasis that is under control, autoimmune thyroid disease or type 1 diabetes. - you have had an inflammation of the lung tissue called
pneumonitis  in the past
in the past - you have urothelial cancer that has spread to the brain unless it has already been controlled with treatment
- you have active HIV. You might be still be able to take part if you have controlled HIV without any symptoms. Your doctor will explain more.
- you have active hepatitis B or active hepatitis C. You might be still be able to take part if you have controlled hepatitis B or hepatitis C without any symptoms. Your doctor will explain more.
- you have had a
live vaccine  within 28 days of joining the trial. You can have the coronavirus (COVID-19) vaccine while having atezolizumab.
within 28 days of joining the trial. You can have the coronavirus (COVID-19) vaccine while having atezolizumab. - you are pregnant or breastfeeding
Trial design
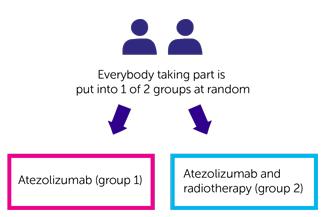
This is a phase 2 trial. The researchers need 102 people from the UK to take part. It is a randomised trial. You are put into treatment groups by a computer. Neither you nor your doctor will be able to decide which group you are in.
You have 1 of the following:
- atezolizumab (group 1)
- atezolizumab and radiotherapy (group 2)
Everyone has atezolizumab as a drip into a vein. The drip takes between 30 minutes and an hour. You have it in the same way as you would if you were having it and not taking part in the trial. This is once every 3 weeks.
If you were having maintenance avelumab before taking part in the trial you stop this before starting atezolizumab.
You have it for up to 2 years or for as long as it is working, and the side effects are manageable.
People in group 2 also have radiotherapy. You have radiotherapy to an area where your cancer has spread to where you have not had radiotherapy before.
You would start radiotherapy during the first cycle of atezolizumab after you have joined the trial. You usually have it Monday to Friday for one week. Each radiotherapy session usually lasts around 10 minutes. But the time you are at the hospital will be longer.
Quality of life
The study team will ask you to fill out questionnaires:
- before you find out which treatment group you are in
- after 9 weeks of atezolizumab (during the trial)
- 6 months after starting treatment on the trial
- a year after starting treatment on the trial
The questionnaires will ask about side effects and how you’ve been feeling.
This is called a quality of life study. You don’t have to take part in the quality of life study. You can still take part in the trial if you say no.
Research samples
The trial team would like to collect some samples for research. They will ask your permission for this. You can say no and still take part in the trial.
The team will collect these when you have your usual blood tests whenever possible.
The researchers would like to collect blood samples:
- before you start trial treatment
- after your radiotherapy treatment (group 2 only)
- 3 weeks after starting atezolizumab treatment on this trial
- 9 weeks after starting atezolizumab treatment on this trial
- if your cancer gets worse (progression)
The researchers would like to use the tissue sample you gave at diagnosis. This is stored at your hospital. You do not have to give another tissue sample.
The trial team aim to use these blood and tissue samples to find out:
- how atezolizumab with and without radiotherapy affects bladder cancer that has spread
- about
genetic  changes and the difference between people’s cancers
changes and the difference between people’s cancers - why some people might develop urothelial cancer
- why treatment might work better for some people than others
Hospital visits
You see a doctor and have some tests before you join the trial. These include:
- a physical examination
- blood tests
- a CT scan
The hospital visits when having atezolizumab are the same if you are taking part in the trial or not. Everyone sees a doctor in clinic before each cycle of atezolizumab. This is around every 3 weeks. At these visits you have:
- a blood test to check your
blood count  and how well your liver and kidneys are working
and how well your liver and kidneys are working - a blood test to see how well your
thyroid  is working – this is once every 6 weeks
is working – this is once every 6 weeks - a chat about your symptoms and how you are managing – with help about how to manage them or treatment if you need any
You have a CT scan to see how well treatment is working:
- every 9 weeks for the first year
- every 12 weeks after the first year
Group 2
You have a planning appointment before starting radiotherapy. This is to work out how much radiation you need and exactly where you need it.
You go to the hospital every day for 5 days in a row for radiotherapy.
Side effects
The trial team monitor you during treatment and afterwards. Contact your advice line or tell your doctor or nurse if any side effects are bad or not getting better.
| Atezolizumab can affect the immune system. It may cause inflammation in different parts of the body. This can cause serious side effects. They could happen during treatment, or some months after treatment has finished. Rarely, these side effects could be life threatening. If you have any of these side effects tell your doctor or nurse as soon as possible. You should tell them that you are on or have been on an immunotherapy. |
The most common side effects of atezolizumab are:
- loss of appetite
- cough
- shortness of breath
- feeling or being sick
- diarrhoea
- urine infection
- rash
- itching
- pain in your muscles, bones or joints including back pain
- high temperature (fever)
- tiredness or lacking energy (fatigue)
- feeling weak
We have more information about the side effects of atezolizumab.
Radiotherapy can cause different side effects depending on which part of the body is being treated. Generally, radiotherapy side effects include:
- tiredness and weakness
- loss of hair in the treatment area
- reddening or darkening of your skin
- skin soreness
Your doctor can explain more about possible side effects once you have your radiotherapy plan.
Atezolizumab could make any side effects of radiotherapy worse. You will be closely monitored during the trial and treatment for side effects can help. Tell you trial team about any side effects that you are having.
We have more information about the general side effects of radiotherapy. And we have more information about the side effects of radiotherapy depending on which part of the body you have treatment to.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Robert Huddart
Supported by
Cancer Research UK
Institute of Cancer Research (ICR)
National Institute for Health Research (NIHR)
Roche
Other information
This is Cancer Research UK trial number CRUKE/19/009.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040