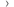A trial looking at hormone therapy with other treatments for prostate cancer (STAMPEDE trial results)
Cancer type:
Status:
Phase:
- had a high risk of the cancer spreading or prostate cancer that has already spread to the
lymph nodes  or elsewhere in the body
or elsewhere in the body - were starting long term hormone therapy for the first time
- were fit enough to have chemotherapy
More about this trial
 in the body. It can work very well, but the cancer often starts to grow again at some stage. Doctors think that having other treatments at the same time as hormone therapy may work better than hormone therapy alone.
in the body. It can work very well, but the cancer often starts to grow again at some stage. Doctors think that having other treatments at the same time as hormone therapy may work better than hormone therapy alone.- a drug called zoledronic acid
- a chemotherapy drug called docetaxel
- an anti inflammatory drug called celecoxib
- another type of hormone therapy called abiraterone
- radiotherapy to the prostate
- another type of hormone therapy called enzalutamide
- a drug called metformin
- a hormone patch called transdermal oestradiol
Summary of results
- adding docetaxel to hormone therapy improves the length of time men live – this is called overall survival
- adding abiraterone to hormone therapy improves overall survival and delays the time until the cancer gets worse
- adding radiotherapy to hormone therapy in men with less prostate cancer spread improves overall survival
- celecoxib doesn’t help men live longer
- adding abiraterone to hormone therapy and radiotherapy could be a new standard treatment option for some men. These are men whose prostate cancer hasn’t spread to another part of the body.
- injections or implants to stop the testicles making testosterone or to block the effects of testosterone
- surgery to remove the testicles or the parts of the testicles that make testosterone - this is called an orchidectomy
- 1,184 had hormone therapy
- 593 had hormone therapy and zoledronic acid
- 592 had hormone therapy and docetaxel
- 593 had hormone therapy, zoledronic acid and docetaxel
- 71 months in men who had hormone therapy
- 81 months in men who had hormone therapy and docetaxel
- 76 months in men who had hormone therapy, zoledronic acid and docetaxel
- an increased risk of infection with a fever
- an increased risk of infection
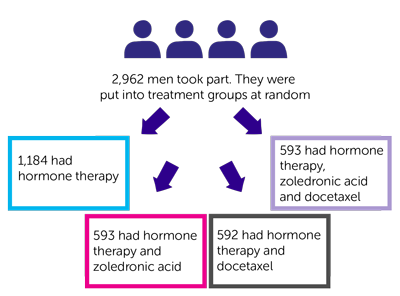
- 957 had hormone therapy
- 960 had hormone therapy, abiraterone and the steroid drug prednisolone
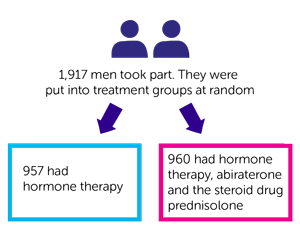
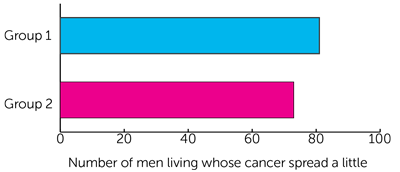
- 7 out 10 men (76%) who had hormone therapy (group 1)
- 8 out of 10 men (83%) who had hormone therapy and abiraterone (group 2)
- 535 men who had hormone therapy
- 248 men who had hormone therapy and abiraterone
- changes in some blood test results
- high blood pressure
- breathing problems
- 622 had hormone therapy
- 312 had hormone therapy and celecoxib
- 311 had hormone therapy, celecoxib and zoledronic acid
- 1,029 had hormone therapy
- 1,032 had hormone therapy and radiotherapy
- 819 men, the cancer had spread a little bit. This is called low metastatic burden. This means it hadn’t spread beyond
lymph nodes  and/or nearby bones
and/or nearby bones - 1,120 men, the cancer had spread further – this is called a high metastatic burden
- 122 men the trial team didn’t have this information
- 8 out of 10 (81%) men who had hormone therapy and radiotherapy (group 1)
- 7 out of 10 (73%) men who had hormone therapy (group 2)
But they found that radiotherapy didn’t help men live longer if their cancer had spread further at the time of diagnosis.
Researchers published these results in 2021. Most of the men had newly diagnosed cancer.
These results looked at men who had prostate cancer that:
- hadn’t spread to another part of the body and
- had a high risk of the cancer spreading
The team analysed the information for 1,974 men. Of those:
- 988 men had hormone therapy alone (group A)
- 986 men had hormone therapy and abiraterone. Some also had enzalutamide (groups G and J)
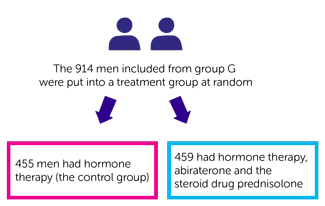
In group G:
- 455 men had hormone therapy (
control group  A)
A) - 459 men had hormone therapy, abiraterone and the steroid drug prednisolone
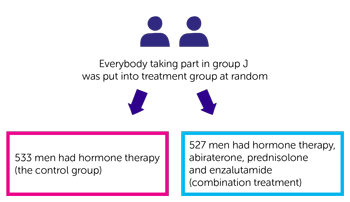
In group J:
- 533 men had hormone therapy (control group A)
- 527 men had hormone therapy, abiraterone, prednisolone and enzalutamide. This is combination treatment.
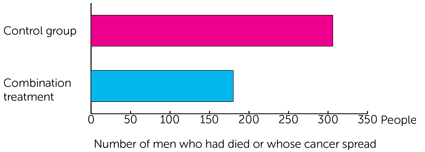
The team followed everyone for about 6 years. They looked at how well treatment worked. To do this they looked at the number of people who had died or whose cancer had spread to another part of the body. At 6 years they found this happened in:
- 306 men who had hormone therapy alone
- 180 men who had hormone therapy and abiraterone, with or without enzalutamide
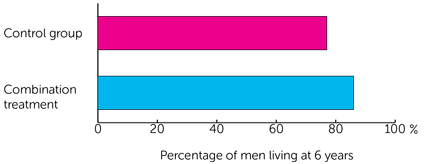
- just under 8 out of 10 men (77%) who had hormone therapy
- just under 9 out of 10 men (86%) who had hormone therapy and abiraterone
Men who had abiraterone had more problems with high blood pressure and mild liver changes. Those who had enzalutamide had more severe problems with high blood pressure, problems getting an erection, tiredness and liver changes.
- docetaxel
- abiraterone which also reduces the chances of the cancer spreading or coming back
- radiotherapy in men with less prostate cancer spread (low metastatic burden)
- abiraterone and prednisolone for men whose cancer hadn’t spread to another part of the body
- celecoxib
- zoledronic acid and adding it to docetaxel didn’t add any extra benefit to having docetaxel and hormone therapy
- adding enzalutamide to abiraterone
 ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Nick James
Supported by
Cancer Research UK
Medical Research Council
Astellas
Clovis Oncology
Janssen
Novartis
Pfizer
Sanofi-Aventis
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040