Colon capsule endoscopy for bowel cancer investigation
On this page
Colon capsule endoscopy (CCE) has the potential to improve patient experience and support endoscopy capacity in UK health systems. Pilots have been initiated across the UK to assess CCE as an initial investigation for people at risk of bowel cancer. Further research and innovation will be required to optimise CCE. While CCE can support diagnosis, colonoscopy is still the gold standard for bowel cancer diagnosis. Find out more about where CCE is at now and what the future may be below.
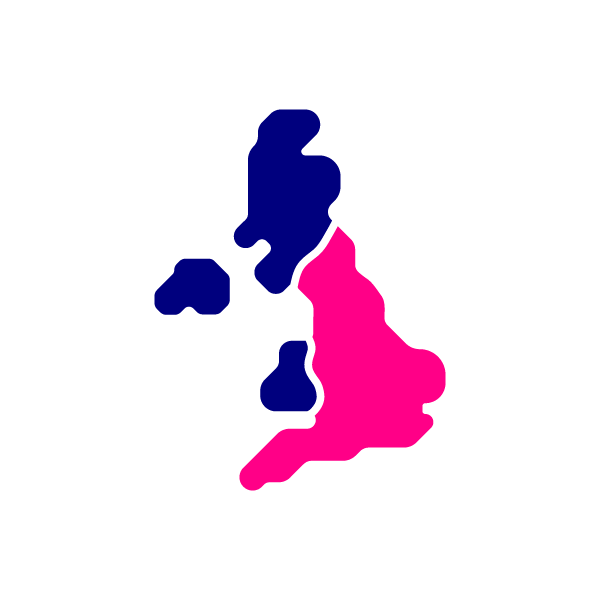
How does CCE work?
CCE pilots in the UK
There have been CCE pilots in health services across the UK, but evidence is still emerging to inform future practice. Below is a summary of the pilots with key results, including signposting links for more information.
Scotland
|
The ScotCAP trial assessed CCE to triage surveillance patients and symptomatic patients with a positive FIT before endoscopy in Scotland.
The trial suggested that CCE was safe and that it could play a role in ruling out further investigation for some patients. After the trial ended in 2020, CCE has been implemented in Scotland to support post-pandemic endoscopy recovery. |
England |
NHS England is trialling CCE for symptomatic patients with an average risk of colorectal cancer (those with a positive FIT result between 10 – 100 ug Hb/g). At some sites, it was assessed for surveillance patients too.
Patient experience will be evaluated as part of the pilot to inform any future roll out. |
Wales |
In 2023, a small CCE pilot was run in Wales across 4 health boards.
Following the pilot, there is interest in embedding CCE as part of a national service in Wales. |
Although CCE is not fully rolled out, use is likely to increase in health services in the future so it’s important to be aware of progress.
Advantages and disadvantages of CCE
Advantages
|
|
Disadvantages |
|
Innovation
Research and innovation will be required to address some of the current challenges with CCE. Future needs include:
- improving battery life
- using AI to speed up image reading time
- better bowel preparation regimen
Research funding has been allocated to assess the feasibility of a new iteration of CCE that will be able to take a biopsy, which would reduce follow-up colonoscopy.
References
If you have any questions about the use of CCE in health systems for cancer diagnosis, get in touch with our Strategic Evidence team.