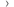ICBP partnership
ICBP newsletters
Updates on research, new findings and relevant international conferences.
About the ICBP
The International Cancer Benchmarking Partnership (ICBP) is a unique and innovative collaboration that brings together clinicians, policymakers, researchers and data experts across the world. It aims to measure international variation in cancer survival, incidence and mortality, as well as identify factors that might be driving these differences.
The ICBP produces high quality research to help identify best international practice, and generate insights needed for policy and practice change. This will help to enable optimisation of cancer services and improvement of outcomes for cancer patients.
Why was the partnership set up?
The ICBP was set up in 2009 to deliver high quality, comparative research on cancer survival, incidence and mortality trends across high income countries. Our research uses a range of data sources to explore factors that could explain international variation in survival rates.
The partnership provides the opportunity to compare data and share practices and policies that seem to be contributing to improved cancer outcomes. The ICBP wants to ensure patients across the globe experience the best possible cancer services and aims to drive international improvement in cancer registry practices.